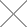What are the Signs of Corrosion?
Instruments installed in process pipelines and production equipment play a crucial role in industrial processes, monitoring and controlling various parameters to ensure safety and stability. However, they can be affected by corrosive media, reducing their performance and reliability over time. In more severe cases, this can lead to issues like leaks, posing safety hazards in the production process.
To detect instrument corrosion issues promptly, we describe some corrosion phenomena in process pipelines, equipment, and instrument bodies. By understanding these signs, we can anticipate and identify corrosion in instruments installed on process pipelines and equipment.
Corrosion Warning One
If you observe pinholes or pitting on metal surfaces, extensive pitting that appears like uniform corrosion, color changes (e.g., black, blue, green) on the metal surface, or surface irregularities such as dents and bumps, it may be due to pitting corrosion.

Pitting corrosion is highly localized and typically starts from surface defects, protruding inclusions, or metal grain boundaries. It often extends below the metal surface to a certain depth and is usually covered by corrosion products, making it challenging to detect. Equipment failure often manifests as isolated or multiple corrosion perforations, with very few cases resulting in complete failure.
In refineries, austenitic, ferritic, and martensitic stainless steels have all encountered pitting corrosion issues. Alloys containing molybdenum can reduce point corrosion in these stainless steels, and materials that exhibit pitting corrosion in corrosion tests should not be used to construct process equipment.
Corrosion Warning Two
Surface or internal cracks in metal materials, characterized by fine, brittle fractures that extend along stress directions, local depressions, dents, or holes of varying depths on the surface, color changes on the surface, increased roughness, and deformations like expansion, contraction, or warping. These signs may indicate stress corrosion cracking.

Chloride-induced stress corrosion cracking (CISCC) occurs when austenitic stainless steel is exposed to chloride ion-containing aqueous environments. It can happen at temperatures between 54°C and 79 °C with low pH or the presence of dissolved oxygen and even trace amounts of chloride ions. Usually, transgranular cracking occurs, but intergranular cracking can also occur.
Austenitic stainless steel can experience cracking in the presence of steam condensate and hot water, and stress corrosion cracking typically requires the presence of water or moisture. Therefore, avoiding alternating wet and dry conditions can to some extent prevent the occurrence of chloride stress corrosion cracking.
If there are numerous small bubbles on the metal surface, typically appearing as round or oval-shaped, varying in size, and they can form densely packed clusters or appear individually, it may be the result of atomic hydrogen diffusing into the steel and getting trapped in gaps, interlayers, or non-metallic inclusions. Hydrogen atoms that enter these areas combine to form molecular hydrogen, which cannot diffuse out. The accumulated hydrogen gas’s expansion pressure eventually leads to wall penetration in the component and the formation of noticeable bubbles on the metal surface.
Additionally, different combinations of metals and corrosive media can result in different stress corrosion manifestations, so specific circumstances may vary.
Corrosion Warning Three
Embrittlement in the grain boundary areas of metals, leading to crack formation along grain boundaries. Pits and holes often form in grain boundary areas, with irregular shapes and varying sizes. Surface discoloration such as blue, purple, brown spots, or areas may also occur, indicating intergranular corrosion.
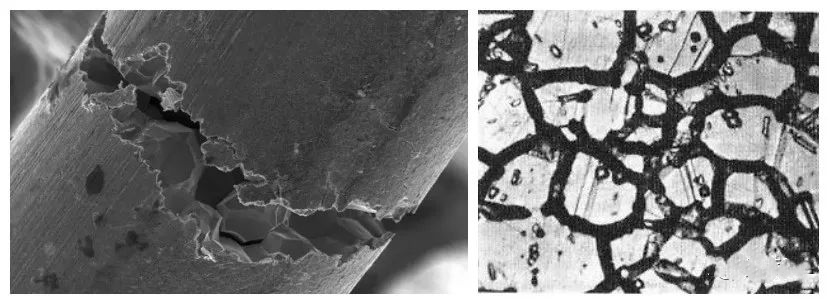
Intergranular corrosion is a highly localized form of corrosion caused by the action of specific chemical environments on metal grain boundaries, leaving the grains themselves uncorroded. It can result in cracking and grain detachment. To prevent intergranular corrosion, proper material selection, optimized processing, or appropriate heat treatment can be employed.
Corrosion Warning Four
Observing electrical currents causing electrochemical reactions on metal surfaces, such as bubble formation or oxidation/reduction reactions on the metal surface. Local corrosion phenomena such as pits, holes, or surface dissolution may occur, as well as changes in color on the surface, such as blue, green, brown spots or areas. These signs could indicate galvanic corrosion.

Galvanic corrosion is an electrochemical corrosion that occurs when two dissimilar metals or alloys are in contact (electrically connected) under conditions with an electrolyte. The more active metal (having a more negative potential) corrodes preferentially. To prevent galvanic corrosion, similar metals can be chosen, or corrosion inhibition methods like coatings or barrier materials can be used.
Corrosion Warning Five
Corrosion, wear, or erosion on the metal surface (indentations, pits, or scars); loss of original smoothness, resulting in roughness or unevenness; increased system noise and vibration; reduced cross-sectional area of gas flow channels, leading to reduced flow or increased pressure drop. These signs may indicate cavitation.
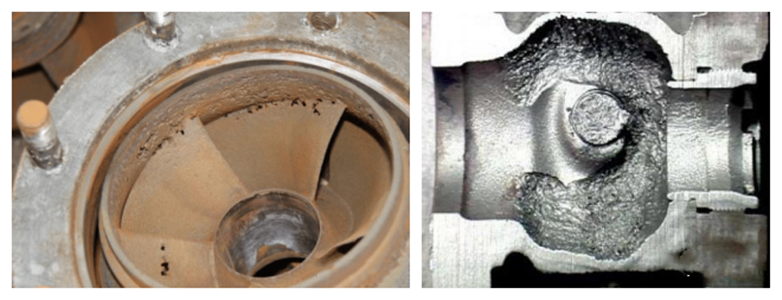
Cavitation effects can be mitigated by choosing materials that resist cavitation, such as stainless steel, nickel alloys, etc. Adjusting fluid temperature, pressure, flow rate, and other parameters can also help. Employing cavitation suppressors, flow regulators, and buffers can be used to reduce cavitation effects.
Corrosion Warning Six
Abnormal increase or decrease in the outer surface temperature of equipment or pipelines, significant temperature differences compared to the surrounding area, depression, cracks, or delamination of insulation layers, liquid or gas leaks, abnormal noise or vibration, changes in appearance such as color changes, surface roughness, or oxidation. These signs may indicate corrosion under insulation.

To mitigate this effect, proper wrapping and sealing to keep the insulation dry can be employed. Before insulation work, apply corrosion-resistant coatings near flanges, valves, and pumps, as these areas are prone to leaks that can wet the insulation. For austenitic stainless steel equipment and pipelines, low chloride-containing insulation materials can be used. Closed-cell foam glass insulation can also be used for austenitic stainless steel equipment and pipelines to reduce the risk.
Corrosion Warning Seven
Changes in the shape, increased dimensions, or exacerbated deformations of components, making them prone to fractures or damage under stress, such as severe sagging of furnace tubes, may indicate creep damage.
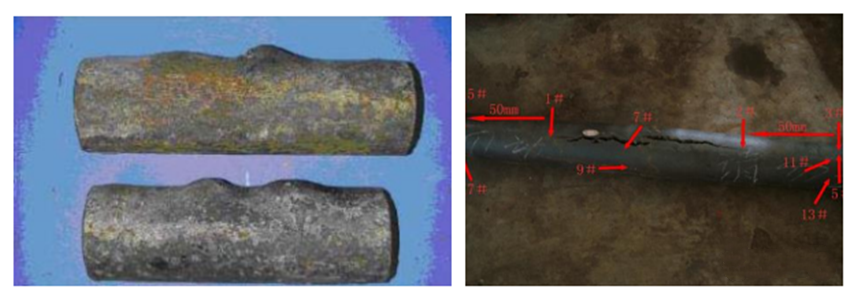
To mitigate creep damage, materials with high creep resistance, such as high-temperature alloys or heat-resistant steels, can be selected. Control the operating temperature and stress of equipment or components to avoid exceeding the material’s creep temperature and stress limits. Reducing the working temperature of equipment or components through cooling systems or other cooling measures can also reduce the risk of creep.
It’s important to note that the manifestation of corrosion may vary depending on factors such as metal materials, temperature, and environmental conditions. Therefore, a comprehensive assessment should be made using multiple observation methods and testing techniques when analyzing corrosion.
By understanding corrosion phenomena and taking appropriate measures, we can effectively protect instrument equipment, extend their service life, reduce maintenance and replacement costs, and improve work efficiency and production benefits.
Looking for the guidance to the use of instrument especially industrial sensors, contact Holykell.